Canadian Pharmacy: Inhalational insulin (Exubera)

The psychology of the superwoman: Health&Care Mall Team
October 12, 2015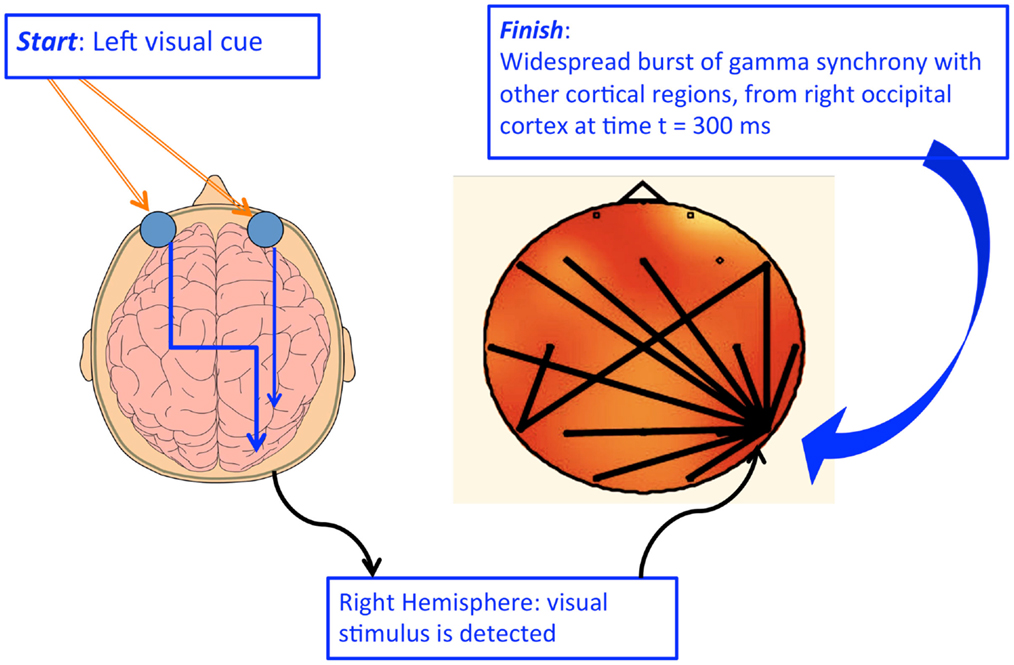
Conscious vision
November 2, 2015Canadian Pharmacy: Inhalational insulin (Exubera)
Inhalation human insulin promises to be rapid-acting and more convenient than traditional insulin injections, but an inhaled product has failed in the past and there are concerns about the potential risks associated with breathing powdered insulin.
Inhalational insulin (Exubera) was approved in 2006 with big expectations but the inhaler was bulky, and patients were put off by the need for periodic lung function tests and eventually it was withdrawn. Afrezza is manufactured by Mannkind Corp and distributed by Sanofi-Aventis. It is the only inhalable insulin and competes with traditional injectable insulin of Eli Lilly and Novo Nordisk.
Afrezza inhalational powder is novel rapid-acting human insulin with a whistle-sized inhaler to control blood-sugar levels in both Type 1 and Type 2 diabetes mellitus (T1DM and T2DM). It was developed in the shadow of inhalational insulin (Exubera) and approved by the U.S. Food and Drug Administration in June, 2014.
DRUG DESCRIPTION
Afrezza is supplied as single-use plastic cartridges filled with dry white powder of human insulin as 4 units or 8 units. The cartridges are color-coded, blue for 4 units and green for 8 units.
The 4 units insulin contains 0.35 mg of insulin and 8 units contain 0.7 mg of insulin. It is administered as single oral-inhalation per cartridge at the beginning of a meal via Afrezza Inhaler only.
The Afrezza Inhaler can be used for up to 15 days from the date of first use. After 15 days of use, Afrezza inhaler must be discarded and replaced with new inhaler. Afrezza contains human insulin produced by DNA recombinant technology utilizing a non-pathogenic laboratory strain of Escherichia coli. Insulin is adsorbed onto carrier particles consisting of fumaryl diketopiperazine and polysorbate 80.
Indications
It has been recently approved by the US Food and Drug Administration and indicated for use in adults ≥18 years of age with T1DM or T2DM. In patients with Type 1 diabetes, must use with a long-acting insulin. It is not a substitute for long-acting insulin.
Dosage
Dose adjustments are needed when switching from insulin to Afrezza. Insulin naïve individuals should start on 4 units of Afrezza at each meal. Those individual using subcutaneous insulin should estimate the total daily insulin dose.
Administer half of the total insulin dose as long-acting basal insulin. Another half of the total insulin dose is divided equally among the three meal insulin dose. Dosing must be individualized based on metabolic needs and glycemic control required. Blood glucose control is carefully monitored in individual requiring higher doses of Afrezza. If glycemic control is not achieved even with higher doses of Afrezza, consider using subcutaneous mealtime insulin.
More about drugs and medications at Canadian Pharmacy website: http://www.canadianhealthcaremalll.com
