Conscious vision
The point has already been made that the retinohypothalamic tract (RHT) transfers electrical signals from the eyes to the biological clock, the SCN. Classically, the only known receptors capable of light perception in the retina were the rods and cones. Recently, however, a third photoreceptor has been identified in the retinas; these are the intrinsically photosensitive retinal ganglion cells (ipRGCs). The function of the ipRGCs is to perceive light that cues the circadian system via the SCN.
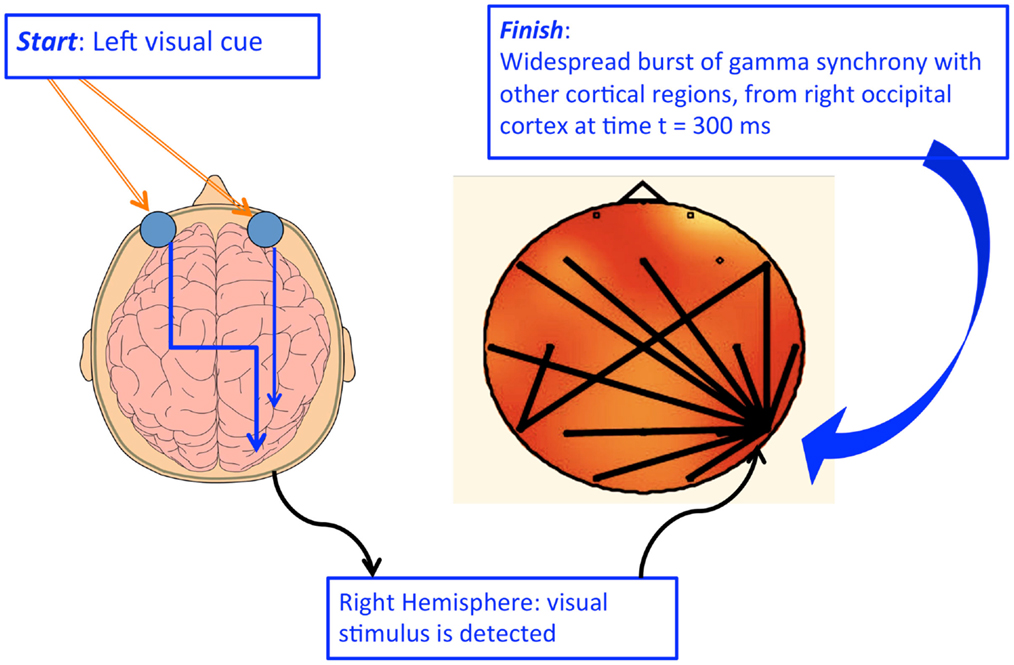
The axons of the ipRGCs project directly or indirectly, via the RHT and other pathways, to the SCN. Conversely, the rods and cones subserve vision and have no direct association with the biological clock. Thus, the retinas of mammals have a dual visual system. These are referred to as “visual vision” (or conscious vision), mediated by classical retinal rods and cones, and “circadian vision” (or unconscious vision) mediated by ipRGCs. The latter is also identified as non-image forming vision.
While circadian vision relies on the ipRGCs, only 1-2% of all ganglion cells are actually capable of responding to light because of the presence of a specialized photopigment, melanopsin, that they contain. This highly specialized and cellimited photopigment is also highly unique in that it is sensitive to only a restricted portion of the usual visible electromagnetic spectrum.
Thus, melanopsin responds primarily to wavelengths in the range of 460-480 nm (blue light). Wavelengths outside of this range, although they influence the rods and cones, are minimally or non-functional in terms of the ipRGGs. Thus, only a limited portion of the visual electromagnetic spectrum is capable of determining the function of the circadian visual system, i.e., regulating the biological clock and endogenous circadian rhythms.
To illustrate the selective independence of the ipRGCs from the remainder of the retina, loss of the retinal rods and cones, e.g., due to their degeneration, minimally alters the ability of light to modulate the SCN and circadian physiology despite the fact that the quantity of melanopsin is dramatically reduced. Hence, an individual can be visually blind (due to loss of the rods and cones) while circadian vision remains intact and functional. Interestingly, however, in melanopsin knock-out mice, the effects of light on the circadian system, based on behavioral parameters, although reduced are not completely lost.
The RHT, axons specifically derived from the ipRGCs, project to the SCN and thereby adjust the circadian activity of the master oscillator. The neurotransmitters in the SCN as well as the molecular mechanism regulating the circadian machinery are, in part, identified. The terminals of ipRGCs in the SCN are not uniformly distributed within these nuclei with the organization exhibiting a remarkable degree of heterogeneity and complexity.
The neural projections from the SCN are highly complex with axons of clock neurons terminating in many areas of the diencephalon. In specific relation to the current review, those axons projecting to the paraventricular nuclei (PVN) of the anterior hypothalamus are of particular interest, given that they are relay nuclei for the photic information being transferred to the pineal gland and determining cyclic melatonin production. The PVN axons project down the brain stem via a non-descript pathway and eventually terminate on preganglionic sympathetic neurons of the intermediolateral cell column of the upper one or two thoracic segments of the spinal cord.
This long descending neural pathway is necessary to link the visual system and the SCN to the peripheral sympathetic outflow of the spinal cord since organs outside the central nervous system that are influenced by the sympathetic division of the autonomic nervous system must receive information from the intermediolateral cell column which is exclusively located in the thoracic and upper lumbar spinal cord. The axons of the preganglionic neurons located in the intermediolateral cell column exist the thoracic cord in the ventral root, ascend in the sympathetic trunk and synapse on postganglionic sympathetic nerve cells in the superior cervical ganglia (SCG).
These ganglia are located at the division of the common carotid into the internal and external carotid arteries. Axons of SCG neurons then accompany the internal carotid arteries and their branches to the tentorium cerebelli where they form rather discrete bundles, the nervi conarii, which penetrate the pineal gland and terminate on pinealocytes, the functional endocrine units of the gland. Surgical removal of the SCG bilaterally sympathetically denervates the pineal gland and renders it incapable of producing melatonin in response to darkness and non-functional in terms of its endocrine actions.